
How many double bonds does this resonance structures have? I need help with the Lewis Dot Structure. Note, that this ion is isoelectronic with CO2. What is the three-dimensional structure of the nuclei of atoms in a molecule or polyatomic ion called? Lewis Dot structure bond length molecular geometry molecular formula Molecular geometry? Lewis Dot structure bond length molecular geometry molecular formula Won't it be Molecular geometry.
#SF2 ELECTRON DOT FORMULA HOW TO#
I am not sure what the formula would be or how to find. Total of valence electrons and the lewis structure. For a lab experiment we have to draw the lewis structures of hydrides. Is that correct? This ion had not been expected to exist, and its instability makes it both diffi cult and dangerous. Formal charge on P is? Give the formal charge on the sulfur atom in a Lewis structure for the sulfate ion in which every atom satisfies the octet rule. What is the formal charge on each of the atoms in the Lewis structure of the PO4 -3 charge?ĭraw another possible Lewis structure of the phosphate ion below. Therefore, ther are two different structures linkage isomers that can be. Both sulfur and nitrogen atoms have lone pair electrons that can potentially be donated. How do you use a Lewis Structure to find the oxidation state of an element. Hydrogen is usually surrounded by 4 electrons in a valid Lewis structure.Ī single bond in a Lewis structure represents 2 electrons. Identify the true statement about Lewis structures. Whatever the case may be, keep learning and keep explaining!H:- If so that is the Lewis dot structure for the hydride ion. We just need to get clarity in mind and focus on the small details which can help us to solve any significant issues of chemistry. Learning about various terms of chemistry just to understand Geometry of Moleculesmakes it a fun learning. I hope you got the answers and detailed explanation regarding Sulfur difluoride. So, SF2 is a polar molecule because of those differences in the electronegativity. We have the sides where there is fluorine, and then there is a side where that lone pair of electrons stays! So, because we have these different sides, this molecule is not symmetrical, and we get a negative as well as a positive side, which makes this a polar molecule. CS2 Lewis Structure, Hybridization, Polarity and Molecular Shape Here what we can see from this structure is that we have two different sides. So as the electron pairs and the fluorine spread out, we end up with this bent geometry. You can see here that we have the two lone pairs on the top and the fluorine atoms are forced down to the bottom. In short, all of these forms the geometry of the Sulfur Fluorine molecule. So according to Valence Shell Electron Pair Repulsion theory VSEPR theorythese fluorine and these two lone pairs of electrons - they are going to spread out, and when they do it, they are going to give us a molecular geometry. We can see that we have two fluorine on either side of the Sulfur and we also have two pairs of lone pair electrons. To determine whether SF2 is polar or nonpolar, first look at the Lewis structure. If we talk about the bond angles, it is 98 which is very similar to H2O. The hybridization by the central Sulfur is SP3. As I have described earlier, the two lone pairs of electron of SF2 gives it a bent shape. Whenever the canter atom has two lone pairs and two particles, the geometry is bent or angular. It forms one bond because it has seven valence electrons and it only needs one more to get to eight. Twenty minus Sixteen So what it tells us is that there are four electrons or two lone pairs of the central sulfur atom and fluorine. Now, when the figure is subtracted, we get four. Now we are going to subtract that sum from the highest multiple of eight but just below twenty, which is obviously sixteen.

This combination gives us the total of twenty.
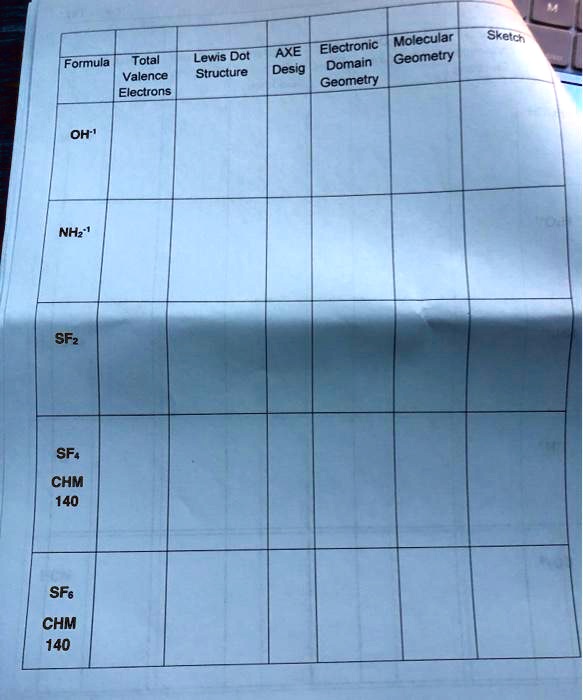
To know about the Sulfur Fluorine - SF2 molecule geometrythe very first thing we have to do is to add up the valence electrons.Īs you may know, Sulfur has six valence electrons, and the Fluorine has seven valence electrons. So in this article, I am going to solve all the confusions regarding of the Sulfur DiFluoride - SF2 molecular geometry. There are so many things to know about such as molecular geometry, Lewis structure, polarity, hybridization, as well as bond angles, but very little information available online. Many of my students were confused after not getting any useful information about SF2 on the internet.


 0 kommentar(er)
0 kommentar(er)
